GMP-Like Production Base Put Into Use & New GMP Grade Restriction Endonuclease Launch
GMP-Like Production Base Put Into Use &
New GMP Grade Restriction Endonuclease Launch
| Production Base Introduction |
 ▶YuGong- GMP-Like Production Base
▶YuGong- GMP-Like Production Base
In order to meet the high standard of raw material quality requirements of users in the fields of mRNA vaccine & drugs, CGT and molecular diagnostics, Yugong GMP-Like production Base has been completed and put into operation in August 2023. The base is located in Lianyungang Area of China (Jiangsu) Pilot Free Trade Zone, the project area is 4,000 m² including 1,200 m² of clean workshop. It has 2 individual production lines, with an annual capacity to meet the raw material demand for mRNA vaccine production for 2 billion dose.

| Quality Standard |
In accordance with the QBD (quality by design) principle, the base layout, equipment selection, employee qualification and other aspects of GMP standards and other relevant regulations. The clean workshop has two individual production lines, each equipped with 100 L fermentor, chromatographic purification system, and all materials are transmitted in closed pipes to minimize exposure and cross-contamination risks. The water purification equipment, air compressor, air conditioner and so on are also put into use after installation, debugging and performance confirmation in accordance with GMP standards.
 ▶Yugong clean workshop
▶Yugong clean workshop
For GMP grade raw protein products, Yugong ensures that the entire production process and raw materials and accessories can be traced, no antibiotics or animal origin materials are used, and the process related impurities such as host residual protein, host residual DNA, non-specific endonuclease, DNase, RNase, as well as microbial contamination and bacterial endotoxins are strictly controlled. So as to meet the requirements of raw materials for vaccine and drug production, cell therapy and other fields.
| New GMP Grade Restriction Endonuclease Launch |
GMP Grade restriction endonuclease for mRNA vaccine production linearizes circular plasmid DNA with antigen-coding genes to generate linear in vitro transcriptional templates.
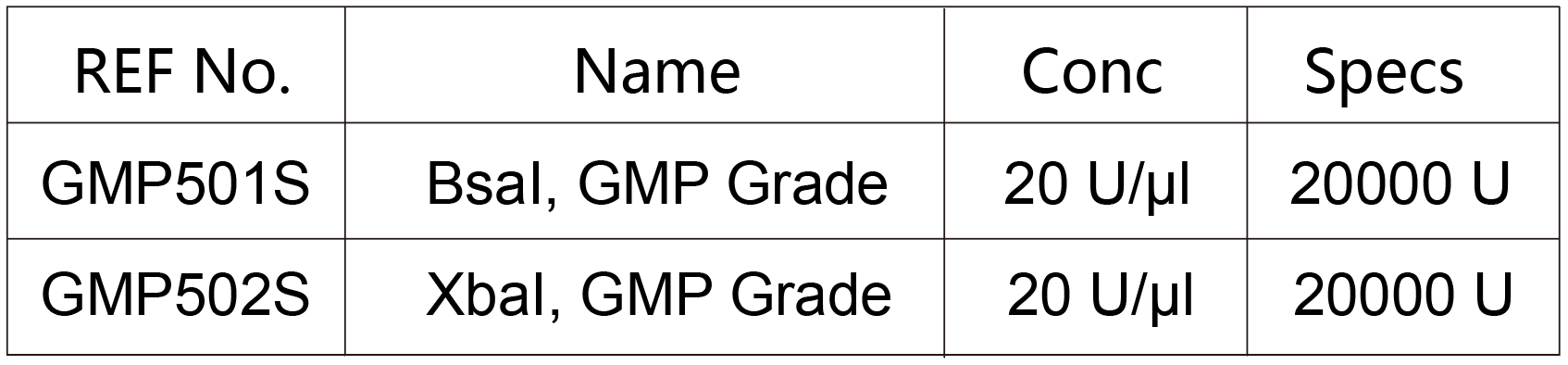
▶BsaI
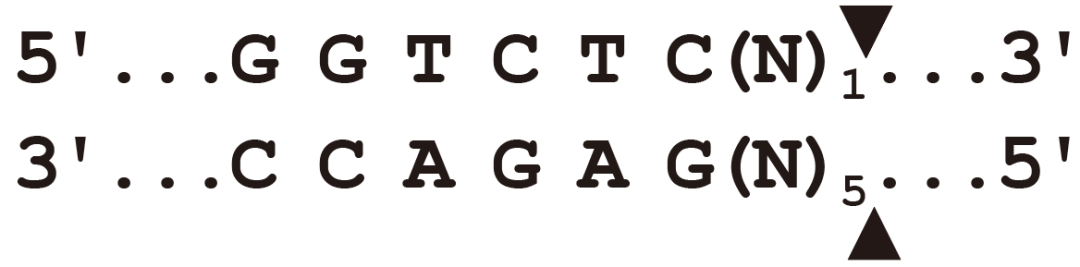
▶XbaI
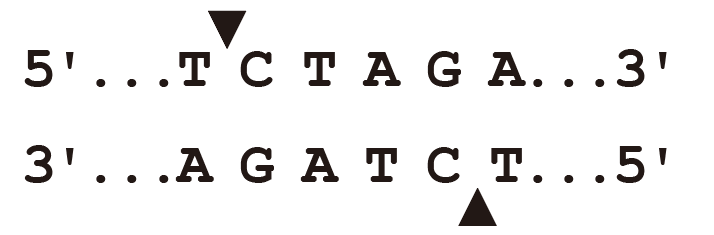
▶Quality Control Assays
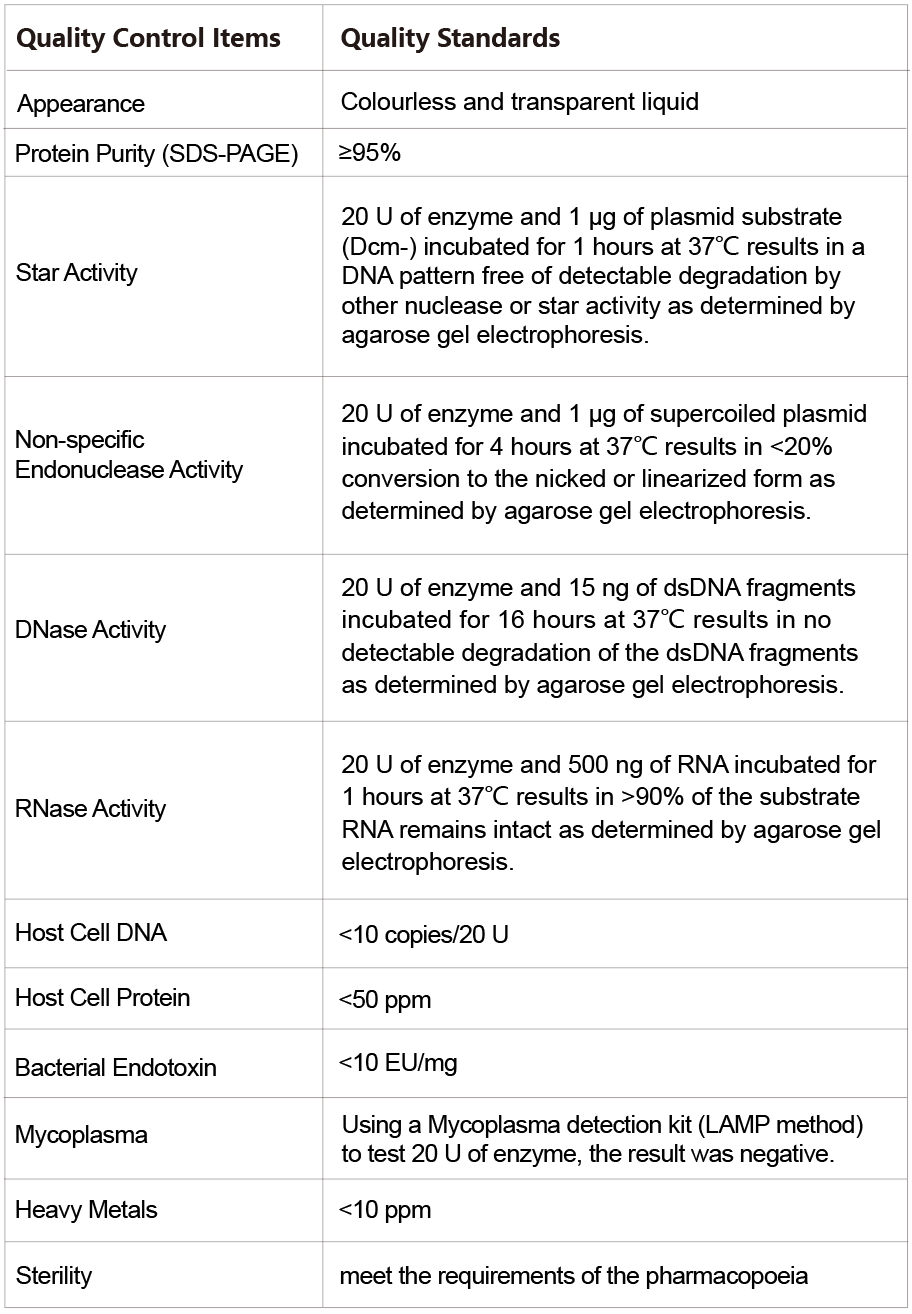
More GMP Grade products will be launched successively

